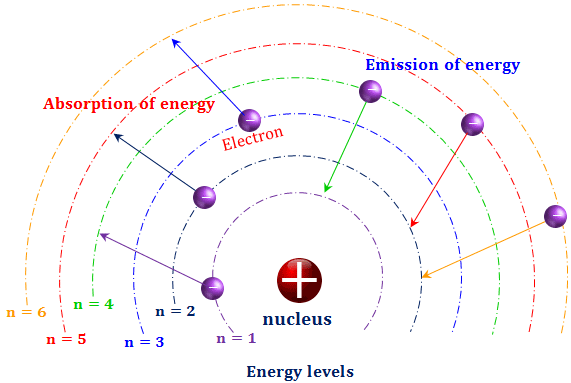
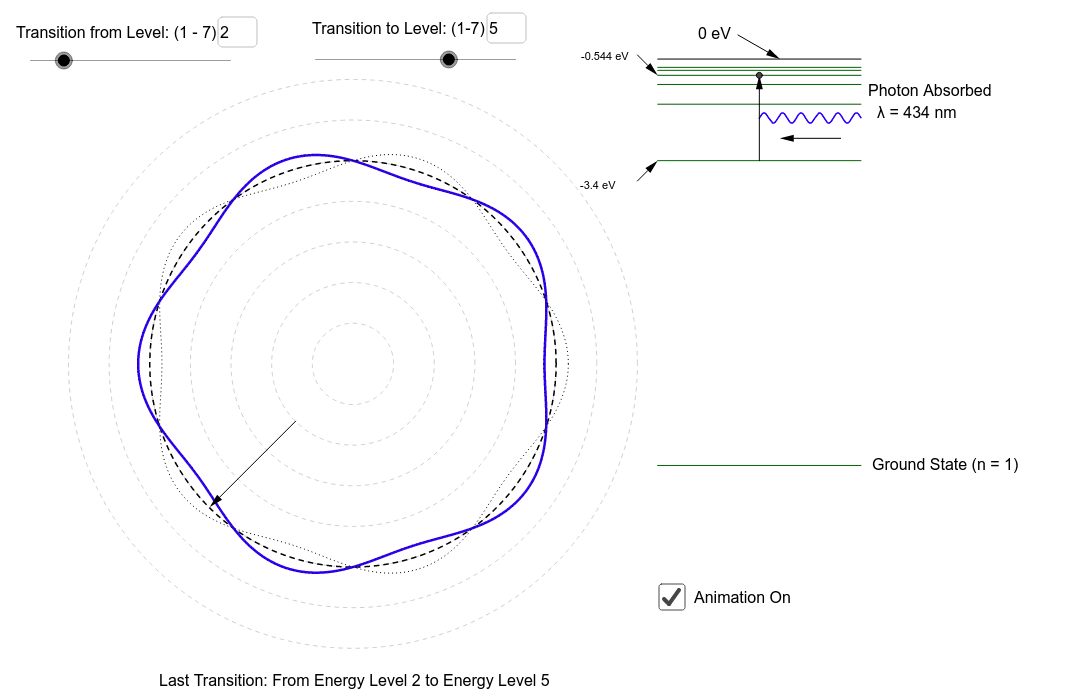
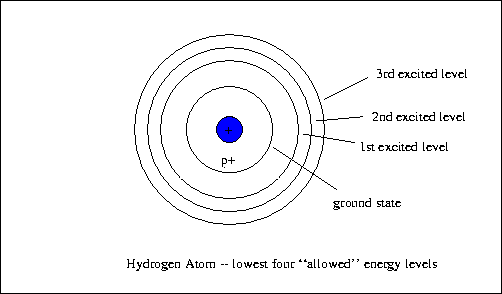
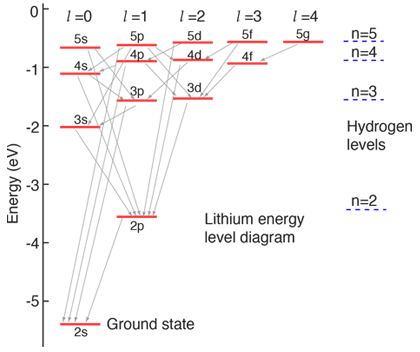
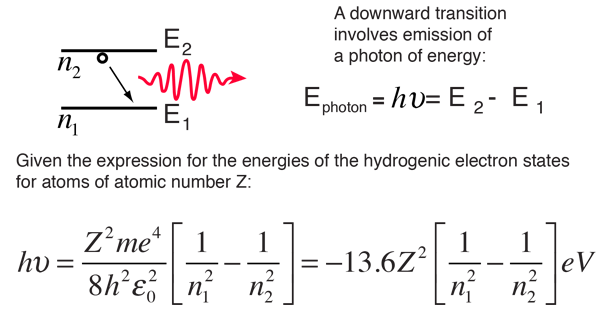
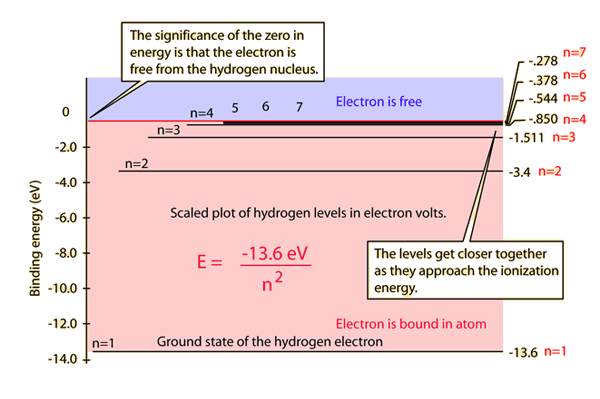
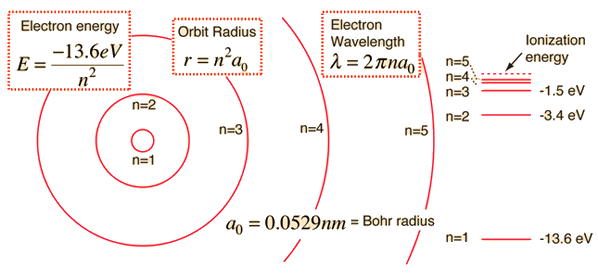
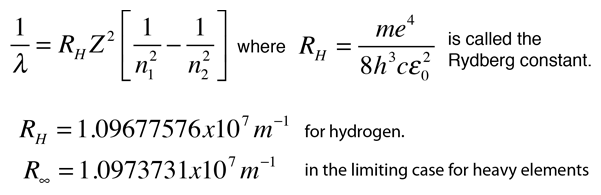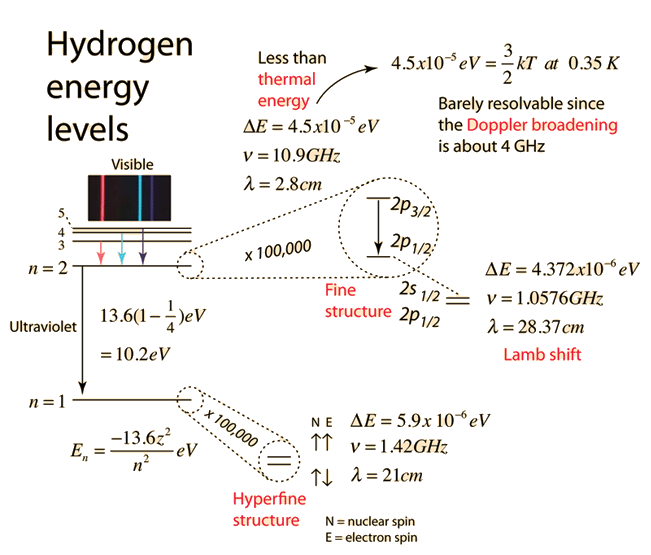The energy of the electron in the ground state of hydrogen atom is - 13.6 eV. Find the kinetic energy and potential energy of electron in this state.

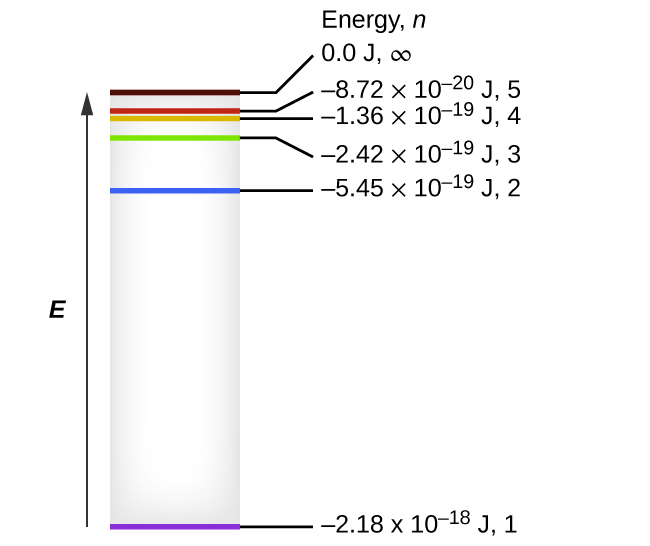
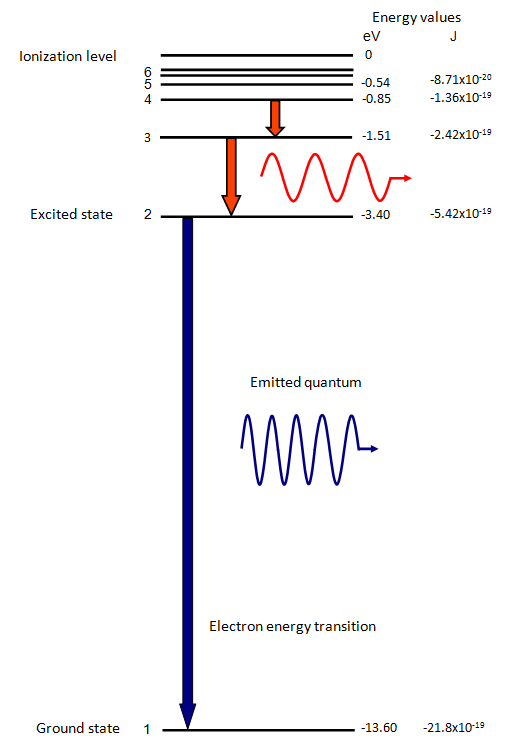
The electron energy of hydrogen atom in the ground state works out to be - 2.18 xx 10^(-18) J per atom. Calcu
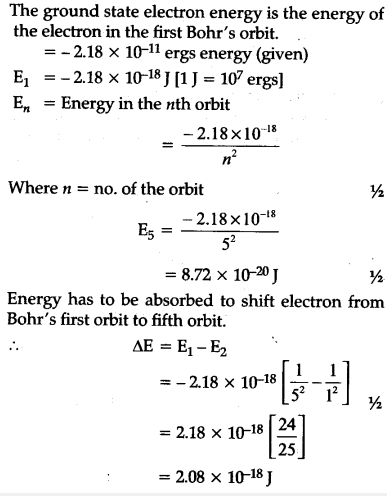
What is the energy in joules, required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit and what is the wavelength of the

The electron energy in hydrogen atom is given by En = (-2.18 x 10-18)/n2 J. Calculate the energy required to remove an electron completely from the n=2 orbit. What is the longest
The electron energy in hydrogen atom is given by En = -217 x 10^-12/n^2 ergs. - Sarthaks eConnect | Largest Online Education Community